AIM: to assess the uniformity of diameter, thickness and hardness of tablets
DATE: 4th December 2014
INTRODUCTION:
Tablet
is one of the most common dosage form available and it is widely exceptable .
Therefore, we need to ensure that the tablets fulfill the standards found in British
Pharmacopoiea and United Pharmacopoiea for exmple uniformity of
diameter, thickness and hardness. Tablet Hardness Tester is a device that can
measure the mechanical integrity of a tablet including diameter, thickness and
hardness of a tablet. The uniformity of these parameters are very important.
This is because too soft tablets can disintegrate in transport, meanwhile too
hard tablets could damage teeth. An acceptable hardness is required and tablet strength testing is
necessary for both, research & development of new formulations, and for
quality control.
METHODOLOGY:
Apparatus and
materials:
•
Mefenamic
acid 500mg tablets
•
Tablet
Testing Instrument (PHARMATEST PTB 311)
Procedure:
- 10 tablets were selected and tested for
uniformity of diameter, thickness and hardness using Tablet Testing
Instrument (PHARMATEST PTB 311).
- The
results were recorded and the deviation
of individual unit from the mean diameter was calculated. The
deviation should not exceed ± 5%
for tablets with diameter of less than 12.5 and ± 3% for diameter of 12.5
mm or more.
RESULTS AND CALCULATIONS:
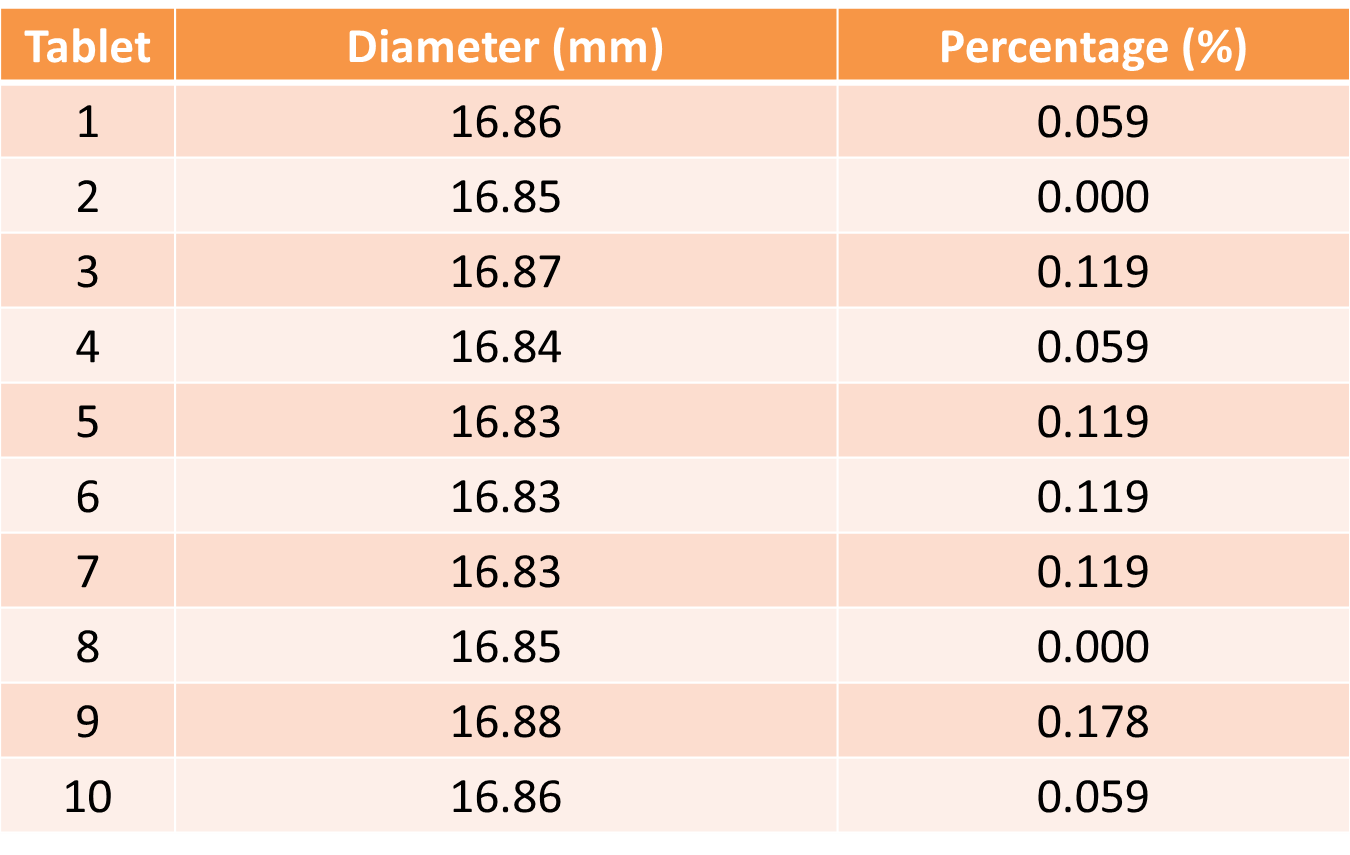
CALCULATION:
Mean of diameter (mm) = Total
diameter of all tablets
10
= (16.86 + 16.85 + 16.87 +
16.84 + 16.83 + 16.83 + 16.83 + 16.85 + 16.88 + 16. 86)
10
10
= 16.85 mm
Percentage
(%) = │ (Experimental diameter – Mean diameter) / Mean diameter│ X 100
DISCUSSION:
In
this experiment, 10 tablets of Mefenamic Acid of 500mg were used to test their
uniformity in diameter, thickness and hardness. Based on the results obtained,
it showed that the thickness and diameter of each tablet were uniform in the
range of 0.57-0.69 mm and 16.83-16.88 mm respectively. This indicates that the
tablets have achieved the uniformity in diameters and thickness.
The
uniformity of diameter of the tablets can be further proven with the percentage
difference. Theoretically, tablets with diameter 12.5 mm or more than 12.5 mm
would have not more than 3% of individual deviation from the average diameter.
From the results obtained, all tablets have percentage deviation below 3%. This
shows that each tablets obeyed the
theoretical value of standard diameter.
Next,
for the hardness of tablets, the result proved that each tablet have its own
hardness. There were three tablets that showed relatively strong hardness which
are tablet 1 (146.6) , tablet 5 (152.1) and tablet 8 (153.5). The tablets must
be hard enough to withstand mechanical stress during packaging, shipment, and
handling by the consumer.
CONCLUSION:
In
pharmaceutical formulation, tablets of the same type have a uniform value in
their diameter and thickness. However, the hardness of the tablets in this
experiment varies. The range of the least strength and the strongest tablet is
between 101.5 to 153.5. This shows a big difference and it can be concluded
that the hardness were not uniform.
REFERENCES:
Dr. Mukesh Gohel. Pharmaceutical Tablets Advantages and Disadvantages. http://www.pharmainfo.net/tablet-ruling-dosage- form-years/what-tablet [ 6 Disember 2014]
Schleuniger. Tablet Hardness
Test. http://www.pharmatron.com/products/hardness- testers/ [6 Disember 2014]
The Pharmaceutics and Compounding Laboratory. http://pharmlabs.unc.edu/labs/tablets/evaluation.htm [6 Disember 2014]







No comments:
Post a Comment